The importance of protein and ligand orientation during 3D visualization
The orientation of protein structures is a simple yet essential
manipulation in any 3D visualization tool for medicinal and
computational chemists. In silico workflows during early-stage
hit and lead identification in drug discovery often rely on the clear
visual representation of interactions between a series of small, bound
ligands and the corresponding active site of the target.
Ligand alignment within Flare
uses Cresset’s proprietary XED force field description of molecules for
fast and effective 3D ligand alignment, as well as more traditional
alignment methods based on maximum common substructure. It is also
possible to superimpose ligands based on a simple selection of atoms.
When working with ligands in the context of proteins, it is often
advantageous to apply the transformation that superimposes two (or more)
bound ligands along with their associated protein structures. This
gives a superimposed view of the proteins that is not achieved by
aligning any of the protein sequences, but by aligning their bound
ligands.
Although Flare does not have the native ability to reorientate proteins based on a superimposition of their bound ligands, the Flare Python API
allows users to create custom features through access to most of
Flare’s functionalities via a Python module. This has the advantage of
providing a higher degree of control over the application beyond
accessing the existing features through the graphical user interface
(GUI). Users can create high throughput workflows or can use the
functionalities to create custom tools. The latter is the focus of the
present article where we demonstrate how a Flare Python API script can
be used to expand the features of the base application.
We present here a simple methodology for reorienting proteins based
on an atom-based ligand alignment, using a script written in the Flare
Python API.
Re-orientate a protein using a ligand superimposition
As an example, a single protein was downloaded from the PDB (4QAC)
and prepared in Flare. This protein is a barrel protein consisting of a
10-fold biological multimer, with individual monomers suitable for
superposition in Flare. Once the protein was prepared, all chains except
for the A- and B-chains were removed along with their associated
ligands in the Flare Ligands Table. The B-chain was then defined as a
separate protein resulting in two objects in the Flare Proteins Table:
one consisting of the original A-chain, and the second protein
consisting of the original B-chain. A snapshot of this is shown in
Figure 1.
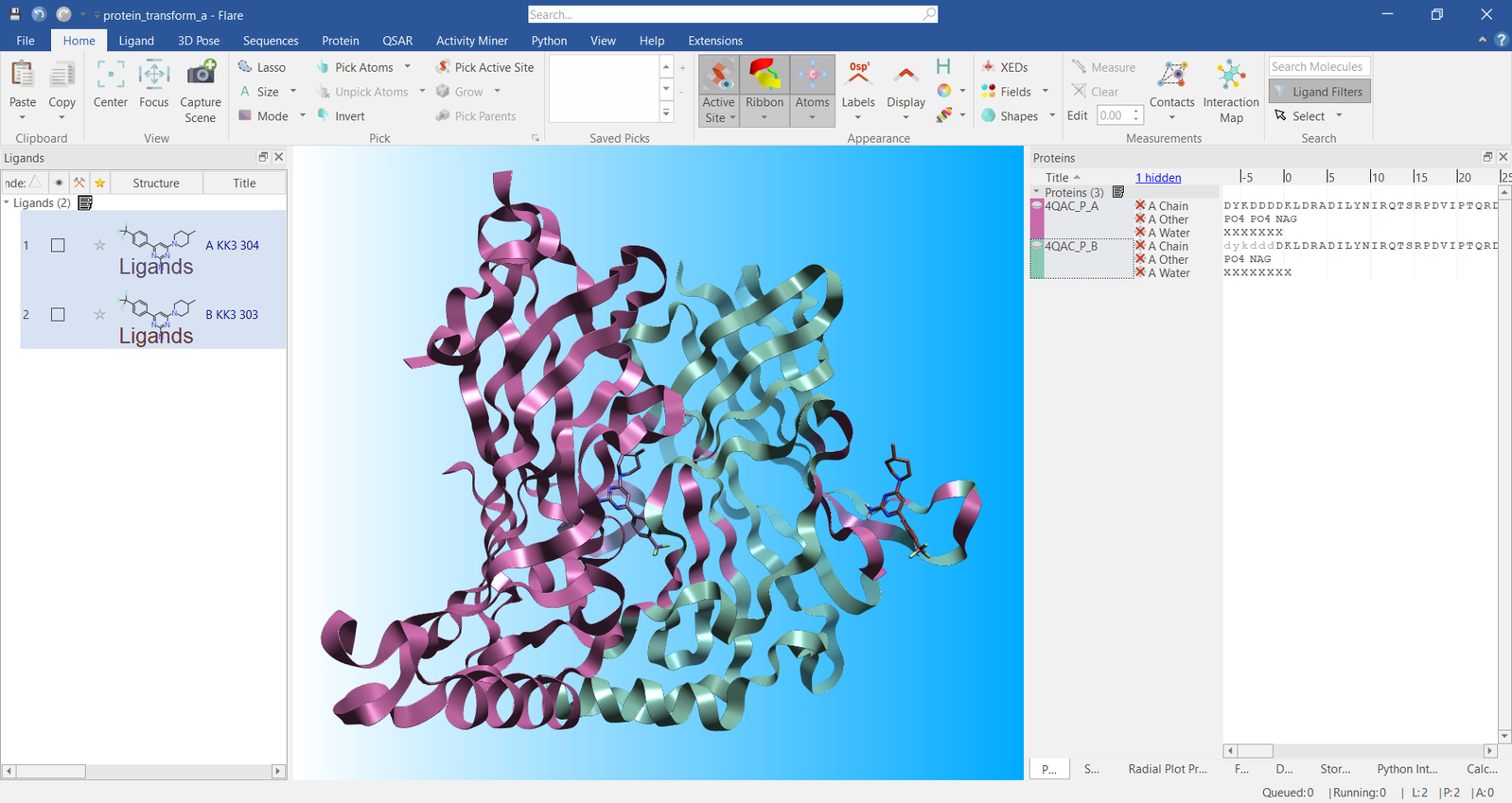 Figure 1. Visualization of the protein A- and B- chains in Flare
Figure 1. Visualization of the protein A- and B- chains in Flare
To begin superimposing the ligands, we first displayed the atom
numbers using the keyboard shortcut, ‘Shift+i’. Figure 2 shows the two
ligands with their atom numbers displayed and also confirms that they
are not spatially overlapped. Flare requires at least three pairs of
atoms on which to perform the ligand superimposition, but more pairs can
make the overall superposition better by helping to define the 3D shape
of the molecule. In this example, we used atoms 10, 8,16, and 24, as
these capture longitudinal and latitudinal geometry for this ligand.

Figure 2. Spatially separated ligand molecules with atom numbers displayed.
Once we chose the atom numbers to align on, the ‘transform_qt.py’
script (which we wrote for this exercise) was loaded into the Flare
Python Interpreter. To do this, we selected the ‘Python’ tab then the
‘Python Interpreter’ option, and then clicked the ‘Load…’ button in the
interpreter to navigate to the script.
Before the script was run, the two ligands and two proteins were
selected by clicking on the desired ligands (two) and proteins (two)
while holding the ’Ctrl’ button. The script was then executed by
clicking the ‘Run’ button; this provided a text box for the user to
enter the atom numbers for the reference and fit molecules.
It is important to note that while the present example is being
performed on the same molecules with two copies of the same protein,
this script also works when superimposing two different ligands in two
different proteins. Atoms with the specified numbers in the text box
will be matched on vertical pairs, regardless of whether (or not) the
atom numbers are the same between ligands. For example, as in Figure 3,
atom 10 in the reference molecule will always be matched with atom 10 on
the fit molecule, even if the ligands are different. It is therefore
essential to always specify the same number of atoms in the reference
box as the fit box, with a minimum of three atom numbers in each box.
Each atom number should be separated by a single space.
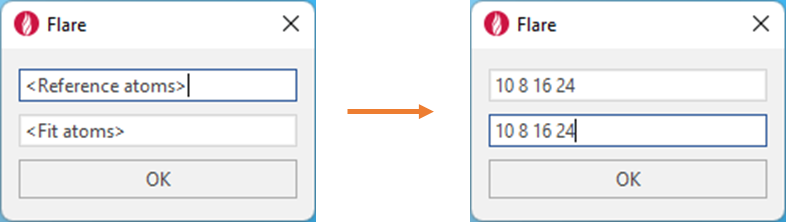
Figure 3. Defining the atom numbers to be matched from the reference molecule to the fit molecule.
The script was run by clicking the ‘OK’
button resulting in the ligands being superimposed onto the atoms
specified. Immediately after the ligand superimposition, the proteins
were superimposed using the same transformation matrix that superimposed
the ligands. The results of this transformation are shown in Figure 4.
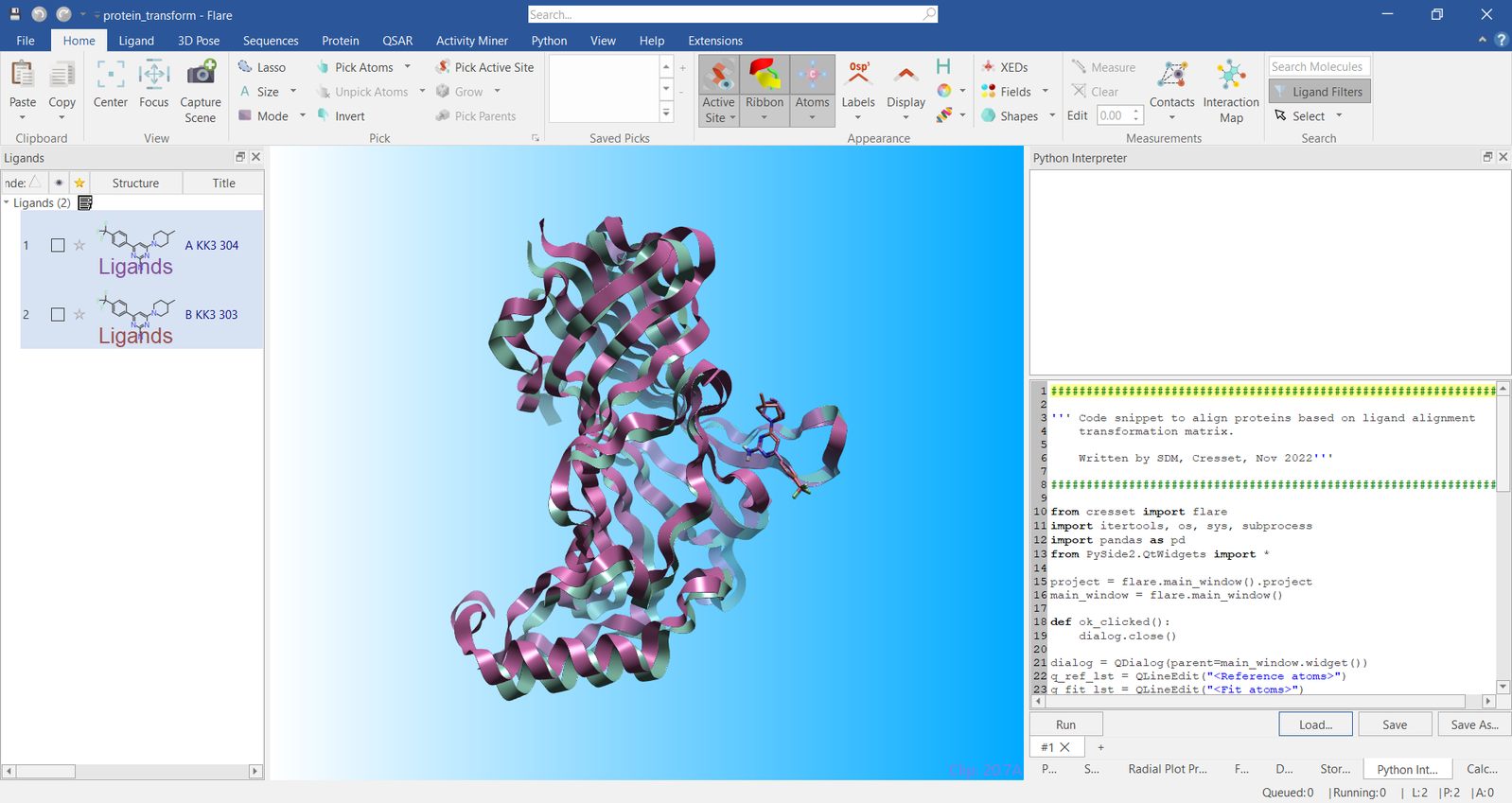
Figure 4. Visualization of the two example proteins transformed by ligand superimposition.
Summary
Flare enables the orientation of proteins to be adjusted by
superimposing their bound ligands. In this article, we have demonstrated
how this can be achieved for two monomers of a biological multimer.
However, this workflow can be used to superimpose
ligand/ligand-protein/protein pairs that are different, provided that
sensible atom pairs can be identified for a ligand superimposition.
For existing Flare customers looking for more information on this
functionality, or seeking to find out how to obtain the Python script
used in this article, please contact Cresset support. If you’re new to Flare and would like to explore the platform’s full range of features in more depth, you can request a free evaluation.